Learning Outcomes
By the end of this lesson, students will be able to:
i. Define and explain the concept of the atom and its significance in understanding matter.
ii. Describe Ernest Rutherford's groundbreaking gold foil experiment and its implications for atomic structure.
iii. Explain the key postulates of Rutherford's atomic model, including the existence of a dense, positively charged nucleus.
iv. Differentiate between Rutherford's atomic model and the earlier plum pudding model proposed by J.J. Thomson.
v. Recognize the limitations of Rutherford's atomic model and its contribution to our evolving understanding of the atom.
Introduction
The atom, the fundamental unit of matter, has captivated scientists for centuries. Ernest Rutherford, a renowned physicist, made a significant breakthrough in our understanding of the atom in 1911. Through his celebrated gold foil experiment, Rutherford challenged existing notions and proposed a revolutionary model of the atom, known as Rutherford's atomic model.
i. The Gold Foil Experiment: A Turning Point in Atomic Understanding
In 1909, Rutherford conducted a series of experiments using a thin gold foil and alpha particles, positively charged helium nuclei. As he bombarded the gold foil with alpha particles, he observed that most particles passed straight through, while a small fraction was deflected at various angles, and a few even bounced back.
These unexpected results contradicted the prevailing plum pudding model, proposed by J.J. Thomson, which depicted the atom as a cloud of positive charge with negatively charged electrons embedded within it. According to this model, alpha particles should have easily passed through the atom without significant deflection.
ii. Rutherford's Atomic Model: A New Perspective on Atomic Structure
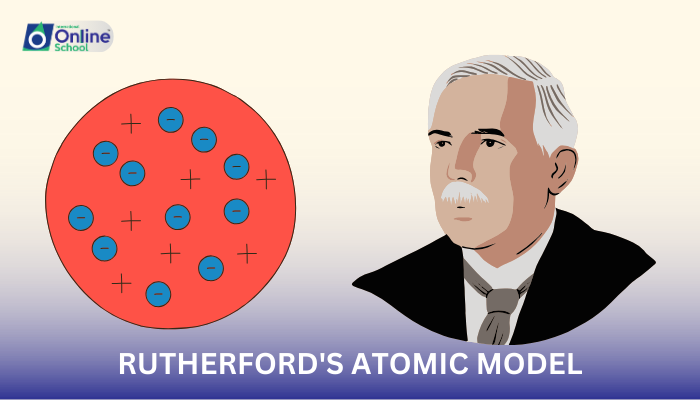
Rutherford's observations led him to propose a new model of the atom, which radically transformed our understanding of atomic structure. The key postulates of Rutherford's atomic model are:
- The atom has a dense, positively charged nucleus at its center. This nucleus, accounting for nearly all the atom's mass, contains protons, positively charged particles.
- Electrons, negatively charged particles, occupy the vast majority of the atom's volume. These electrons are not embedded within the nucleus but rather revolve around it in orbits or paths.
- The positive charge of the nucleus is balanced by the negative charge of the electrons. This balance ensures that the atom remains electrically neutral.
iii. Contrasting Rutherford's Model with Thomson's Model
Rutherford's model contrasted sharply with Thomson's plum pudding model in several ways:
Nucleus vs. Diffused Positive Charge: Rutherford proposed a dense, localized nucleus, while Thomson's model had a diffuse positive charge throughout the atom.
Electron Orbits vs. Embedded Electrons: Rutherford suggested electrons revolved around the nucleus, while Thomson's model had electrons embedded within the positive charge.
iv. Limitations of Rutherford's Model
While Rutherford's model marked a significant step forward, it had certain limitations:
Unexplained Stability of Electron Orbits: Rutherford's model could not explain why electrons revolving around the nucleus did not emit radiation and spiral into the nucleus.
Inconsistency with Atomic Spectra: Rutherford's model did not account for the discrete spectral lines emitted by atoms, which are characteristic of their unique energy levels.
Rutherford's atomic model, despite its limitations, revolutionized our understanding of atomic structure. It introduced the concept of a dense, positively charged nucleus and orbiting electrons, paving the way for further advancements in atomic physics. While subsequent models refined and expanded upon Rutherford's model, his contributions remain fundamental to our understanding of the atom, the building block of the universe.